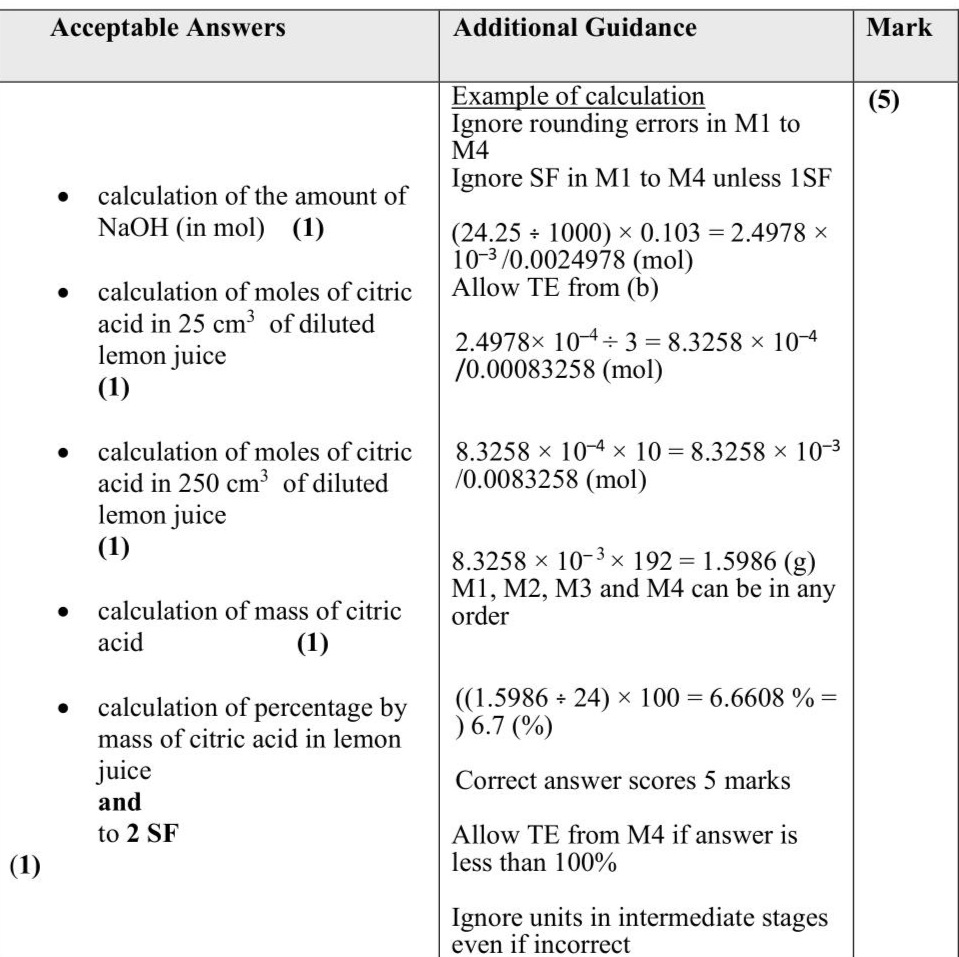
An experiment was carried out to determine the concentration of citric acid in lemon juice using a titration. Three students used the following procedure. Procedure Step 1 Add $$\(24.0 \mathrm{~g}\)$$ of lemon juice to a $$\(250 \mathrm{~cm}^{3}\)$$ volumetric flask. Step 2 Make up the volume of the lemon juice to $$\(250 \mathrm{~cm}^{3}\)$$ using deionised water and mix thoroughly. Step 3 Pipette $$\(25.0 \mathrm{~cm}^{3}\)$$ of the diluted lemon juice into a conical flask and add a few drops of phenolphthalein indicator. Step 4 Titrate the diluted lemon juice with standardised sodium hydroxide of concentration $$\(0.103 \mathrm{~mol} \mathrm{dm}^{-3}\)$$. Student $$\(\mathbf{A}\)$$ obtained the results shown. The equation for the reaction between citric acid and sodium hydroxide solution is shown. $$\[ \mathrm{C}_{6} \mathrm{H}_{8} \mathrm{O}_{7}(\mathrm{aq})+3 \mathrm{NaOH}(\mathrm{aq}) \rightarrow \mathrm{Na}_{3} \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{O}_{7}(\mathrm{aq})+3 \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \]$$ (i) State the colour change that occurs at the end-point of the titration. (1) ................................................................................................................................................................................................................................................................................ (ii) Calculate the percentage by mass of citric acid in the lemon juice, using your mean titre from (b). Give your answer to two significant figures. $$\[ \text { [Concentration of } \mathrm{NaOH}(\mathrm{aq})=0.103 \mathrm{~mol} \mathrm{dm}^{-3} \quad M_{\mathrm{r}} \text { of citric acid }=192 \text { ] } \]$$ (5)
Exam No:wch13-01-que-20240123 Year:2024 Question No:2(c)
Answer:
An answer that makes reference to the following point:
- colourless to (pale) pink
- colourless to (pale) pink

Knowledge points:
1.Formulae, Equations and Amount of Substance
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
