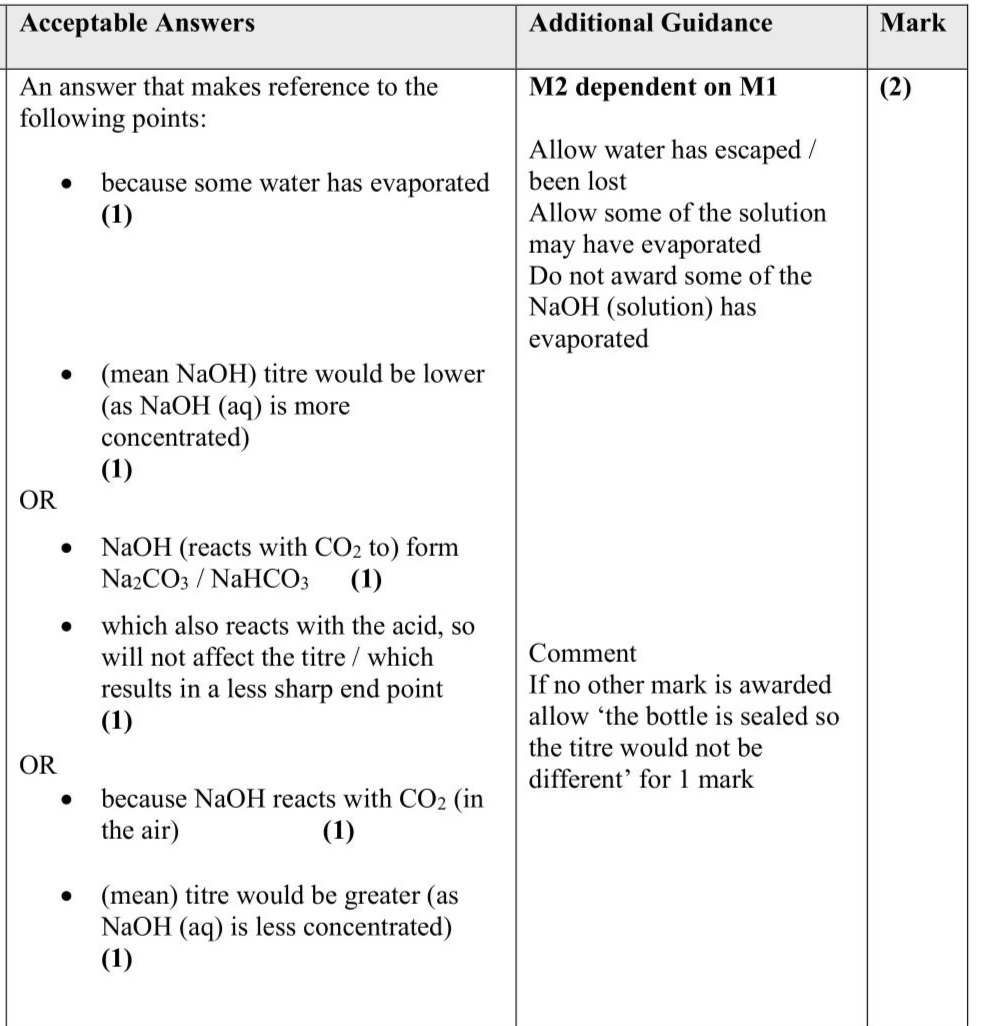
An experiment was carried out to determine the concentration of citric acid in lemon juice using a titration. Three students used the following procedure. Procedure Step 1 Add $$\(24.0 \mathrm{~g}\)$$ of lemon juice to a $$\(250 \mathrm{~cm}^{3}\)$$ volumetric flask. Step 2 Make up the volume of the lemon juice to $$\(250 \mathrm{~cm}^{3}\)$$ using deionised water and mix thoroughly. Step 3 Pipette $$\(25.0 \mathrm{~cm}^{3}\)$$ of the diluted lemon juice into a conical flask and add a few drops of phenolphthalein indicator. Step 4 Titrate the diluted lemon juice with standardised sodium hydroxide of concentration $$\(0.103 \mathrm{~mol} \mathrm{dm}^{-3}\)$$. Student $$\(\mathbf{A}\)$$ obtained the results shown. Two other students, B and C, also followed the procedure to find the concentration of citric acid in similar samples of lemon juice. (i) Student B added too much deionised water in Step 2. State how Student B should correct this mistake. (1) ................................................................................................................................................................................................................................................................................ ................................................................................................................................................................................................................................................................................ ................................................................................................................................................................................................................................................................................ (ii) Student $$\(\mathbf{C}\)$$ used sodium hydroxide solution labelled $$\(0.103 \mathrm{~mol} \mathrm{dm}^{-3}\)$$ that had been made up several months ago and stored since then. Explain what effect this would have on the mean titre, compared to Student A. (2) ................................................................................................................................................................................................................................................................................ ................................................................................................................................................................................................................................................................................ ................................................................................................................................................................................................................................................................................
Exam No:wch13-01-que-20240123 Year:2024 Question No:2(e)
Answer:
An answer that makes reference to the following point:
- (pour away solution, rinse flask and) make a new / fresh solution (of diluted lemon juice)
- (pour away solution, rinse flask and) make a new / fresh solution (of diluted lemon juice)

Knowledge points:
1.Formulae, Equations and Amount of Substance
8.Redox Chemistry and Groups 1, 2 and 7
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
