Compounds $$\(\mathbf{A}, \mathbf{B}, \mathbf{C}\)$$ and $$\(\mathbf{D}\)$$ all have the molecular formula $$\(\mathrm{C}_{4} \mathrm{H}_{8}\)$$. $$\(\mathbf{A}, \mathbf{B}\)$$ and $$\(\mathbf{C}\)$$ each contain one double bond, but $$\(\mathbf{D}\)$$ does not. $$\(\mathbf{A}\)$$ and $$\(\mathbf{B}\)$$ are geometric isomers of each other. Deduce a possible structure and name for each compound. (4)
Exam No:wch11-01-que-20240111 Year:2024 Question No:18(a)
Answer:
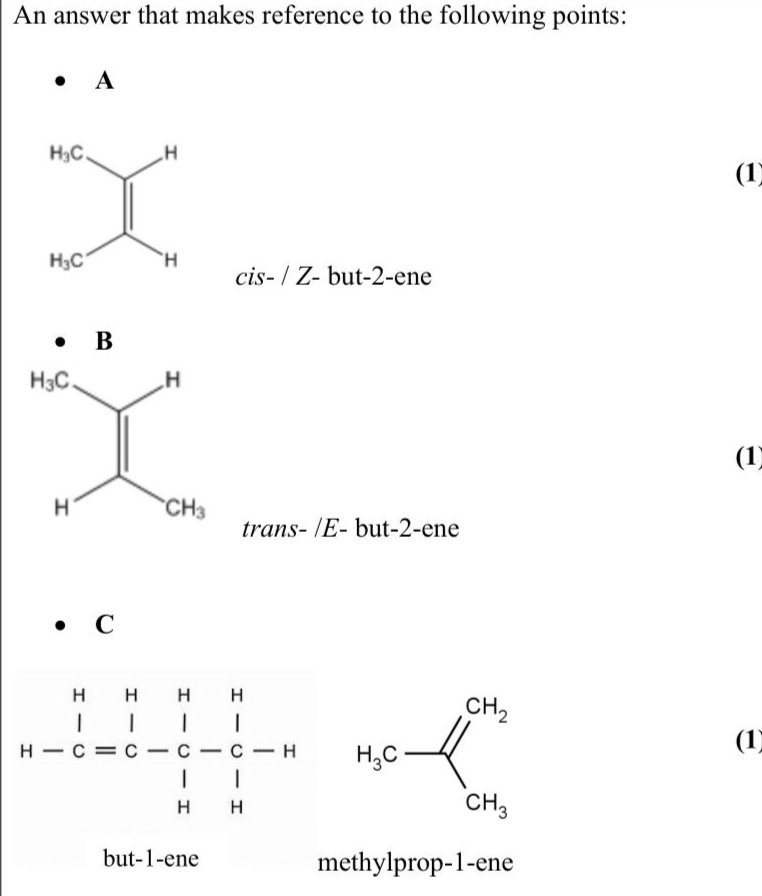
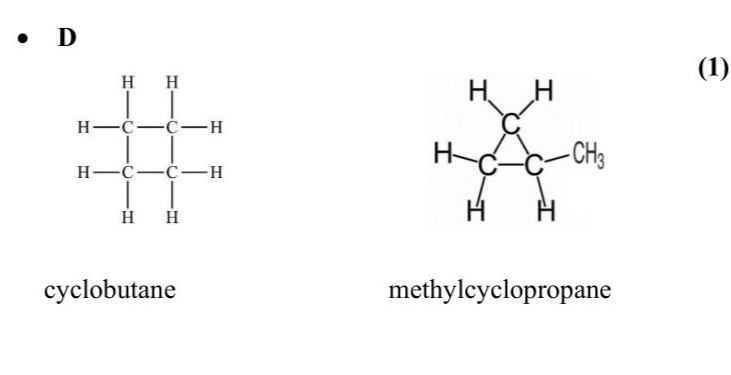
Knowledge points:
5.Alkenes
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
