FA 7 is a solution containing four ions, three of which are listed in the Qualitative analysis notes. Carry out the following tests and record your observations in Table 3.2. Use a 1 cm depth of FA 7 for each test. A boiling tube must be used for Test 1 and a test-tube for the other tests. (ii) Give the formula of each of the four ions in FA 7. The ions are , , and (iii) Give the ionic equation for one reaction that takes place in Test 1 or Test 3 of (b)(i). Include state symbols.
Exam No:9701_m25_qp_33 Year:2025 Question No:3(b)
Answer:

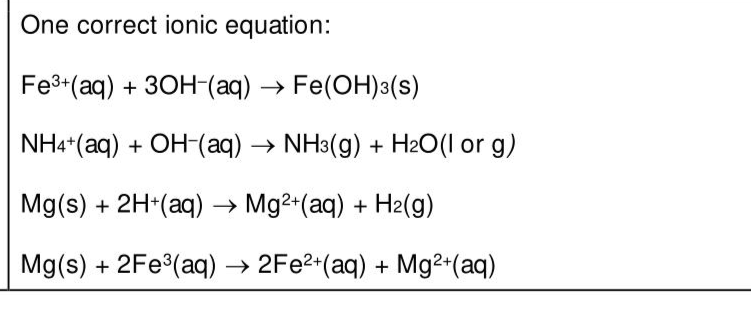
Knowledge points:
10.1.1 describe, and write equations for, the reactions of the elements with oxygen, water and dilute hydrochloric and sulfuric acids
10.1.2 describe, and write equations for, the reactions of the oxides, hydroxides and carbonates with water and dilute hydrochloric and sulfuric acids
10.1.3 describe, and write equations for, the thermal decomposition of the nitrates and carbonates, to include the trend in thermal stabilities
10.1.4 describe, and make predictions from, the trends in physical and chemical properties of the elements involved in the reactions in 10.1.1 and the compounds involved in 10.1.2, 10.1.3 and 10.1.5
10.1.5 state the variation in the solubilities of the hydroxides and sulfates
11.2.1 describe the relative reactivity of the elements as oxidising agents
11.2.2 describe the reactions of the elements with hydrogen and explain their relative reactivity in these reactions
11.2.3 describe the relative thermal stabilities of the hydrogen halides and explain these in terms of bond strengths
11.3.1 describe the relative reactivity of halide ions as reducing agents
11.3.2.1 aqueous silver ions followed by aqueous ammonia (the formation and formula of the $\left[\mathrm{Ag}\left(\mathrm{NH}_{3}\right)_{2}\right]^{+}$ complex is not required)
11.3.2.2 concentrated sulfuric acid, to include balanced chemical equations
6.1.1 calculate oxidation numbers of elements in compounds and ions
6.1.2 use changes in oxidation numbers to help balance chemical equations
6.1.3 explain and use the terms redox, oxidation, reduction and disproportionation in terms of electron transfer and changes in oxidation number
6.1.4 explain and use the terms oxidising agent and reducing agent
6.1.5 use a Roman numeral to indicate the magnitude of the oxidation number of an element
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
