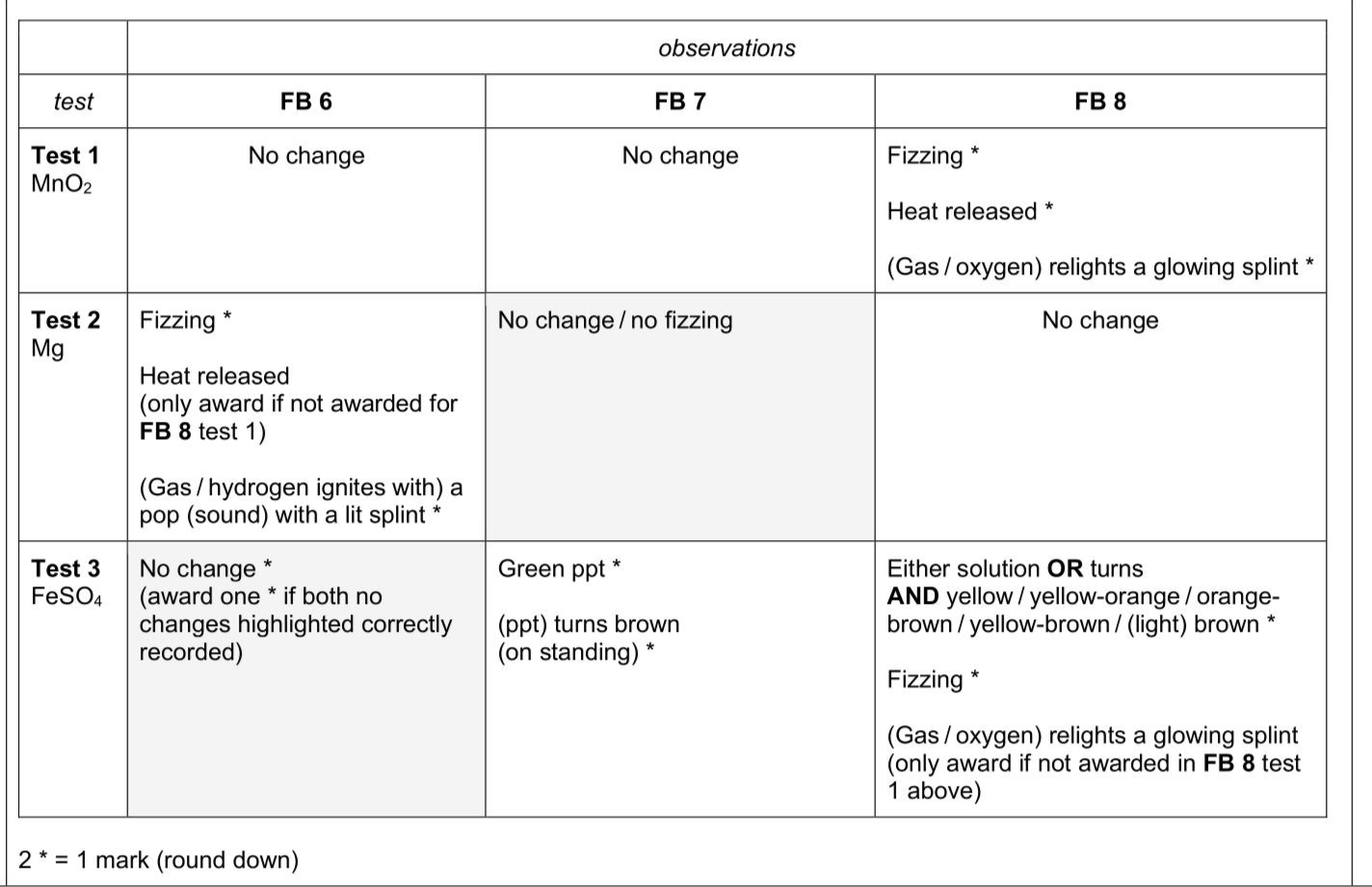
FB 6, FB 7 and FB 8 are aqueous solutions of different compounds that each contain at least one oxygen atom. Carry out the following tests and record your observations in Table 3.1. Three of the tests have been done for you. Use a 1 cm depth of solution in a test-tube for each test. (ii)Use your observations in Table 3.1 to suggest a possible formula for each of FB 6,FB 7 and FB 8. FB 6 FB 7 FB 8 [2]
Exam No:9701_s25_qp_34 Year:2025 Question No:3(a)
Answer:

Knowledge points:
11.3.1 describe the relative reactivity of halide ions as reducing agents
11.3.2.1 aqueous silver ions followed by aqueous ammonia (the formation and formula of the $\left[\mathrm{Ag}\left(\mathrm{NH}_{3}\right)_{2}\right]^{+}$ complex is not required)
11.3.2.2 concentrated sulfuric acid, to include balanced chemical equations
12.1.1 explain the lack of reactivity of nitrogen, with reference to triple bond strength and lack of polarity
12.1.2.1 the basicity of ammonia, using the Brønsted–Lowry theory
12.1.2.2 the structure of the ammonium ion and its formation by an acid–base reaction
12.1.2.3 the displacement of ammonia from ammonium salts by an acid–base reaction
12.1.3 state and explain the natural and man-made occurrences of oxides of nitrogen and their catalytic removal from the exhaust gases of internal combustion engines
12.1.4 understand that atmospheric oxides of nitrogen (NO) can react with unburned hydrocarbons to form peroxyacetyl nitrate, PAN, which is a component of photochemical smog
12.1.5 describe the role of NO in the formation of acid rain both directly and in their catalytic role in the oxidation of atmospheric sulfur dioxide
18.1.1.1 oxidation of primary alcohols and aldehydes with acidifiedor acidified and refluxing
18.1.1.2 hydrolysis of nitriles with dilute acid or dilute alkali followed by acidification
18.1.1.3 hydrolysis of esters with dilute acid or dilute alkali and heat followed by acidification
18.1.2.1 the redox reaction with reactive metals to produce a salt and
18.1.2.2 the neutralisation reaction with alkalis to produce a salt and
18.1.2.3 the acid–base reaction with carbonates to produce a salt
18.1.2.4 esterification with alcohols with concentrated as catalyst
18.1.2.5 reduction by form a primary alcohol
6.1.1 calculate oxidation numbers of elements in compounds and ions
6.1.2 use changes in oxidation numbers to help balance chemical equations
6.1.3 explain and use the terms redox, oxidation, reduction and disproportionation in terms of electron transfer and changes in oxidation number
6.1.4 explain and use the terms oxidising agent and reducing agent
6.1.5 use a Roman numeral to indicate the magnitude of the oxidation number of an element
7.2.1 state the names and formulae of the common acids, limited to hydrochloric acid, HC/, sulfuric acid, ethanoic acid,
7.2.10 select suitable indicators for acid-alkali titrations, given appropriate data
7.2.2 state the names and formulae of the common alkalis, limited to sodium hydroxide, NaOH, potassium hydroxide, KOH, ammonia,
7.2.3 describe the Brønsted–Lowry theory of acids and bases
7.2.4 describe strong acids and strong bases as fully dissociated in aqueous solution and weak acids and weak bases as partially dissociated in aqueous solution
7.2.5 appreciate that water has pH of 7, acid solutions pH of below 7 and alkaline solutions pH of above 7
7.2.6 explain qualitatively the differences in behaviour between strong and weak acids including the reaction with a reactive metal and difference in pH values by use of a pH meter, universal indicator or conductivity
7.2.7 understand that neutralisation reactions occur when
7.2.8 understand that salts are formed in neutralisation reactions
7.2.9 sketch the pH titration curves of titrations using combinations of strong and weak acids with strong and weak alkalis
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
