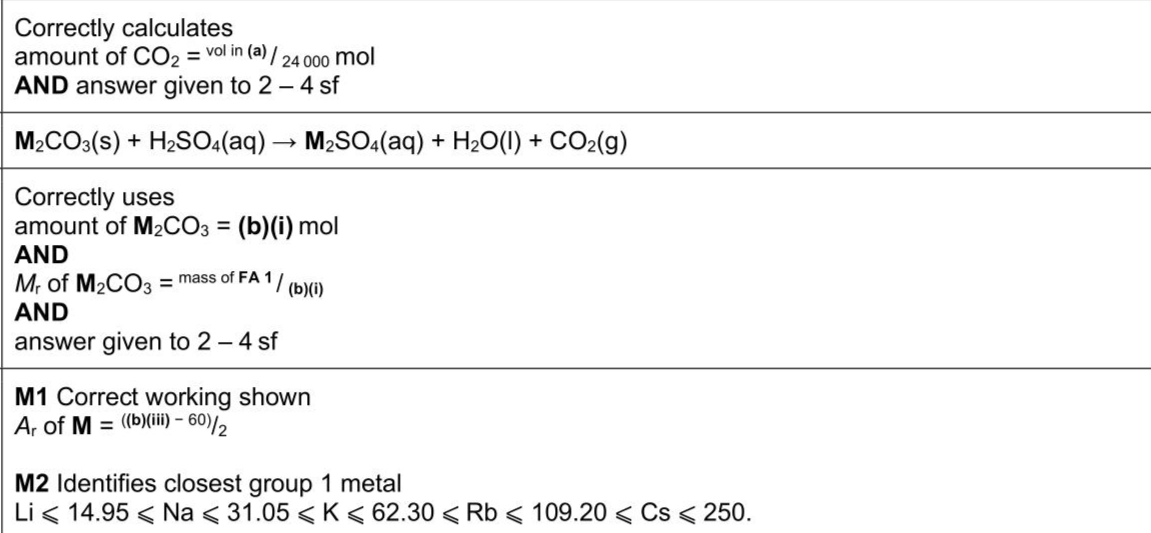
Group 1 metal carbonates react with dilute acids to form a salt, water and carbon dioxide. The identity of the metal, $$\(\mathbf{M}\)$$, can be determined by measuring the volume of carbon dioxide produced when excess acid is added to a known mass of the carbonate, $$\(\mathbf{M}_{2} \mathbf{C O}_{3}\)$$. FA 1 is the metal carbonate, $$\(\mathrm{M}_{2} \mathrm{CO}_{3}\)$$. FA 2 is $$\(0.250 \mathrm{~mol} \mathrm{dm}^{-3}\)$$ sulfuric acid, $$\(\mathrm{H}_{2} \mathrm{SO}_{4}\)$$.
Exam No:9701_s25_qp_35 Year:2025 Question No:1(b)
Answer:
Knowledge points:
10.1.1 describe, and write equations for, the reactions of the elements with oxygen, water and dilute hydrochloric and sulfuric acids
10.1.2 describe, and write equations for, the reactions of the oxides, hydroxides and carbonates with water and dilute hydrochloric and sulfuric acids
10.1.3 describe, and write equations for, the thermal decomposition of the nitrates and carbonates, to include the trend in thermal stabilities
10.1.4 describe, and make predictions from, the trends in physical and chemical properties of the elements involved in the reactions in 10.1.1 and the compounds involved in 10.1.2, 10.1.3 and 10.1.5
10.1.5 state the variation in the solubilities of the hydroxides and sulfates
2.2.1 define and use the term mole in terms of the Avogadro constant
2.3.1.1 the prediction of ionic charge from the position of an element in the Periodic Table
2.3.1.2 recall of the names and formulae for the following ions:
2.3.2 write and construct equations (which should be balanced), including ionic equations (which should not include spectator ions)
2.3.3 use appropriate state symbols in equations
2.3.4 define and use the terms empirical and molecular formula
2.3.5 understand and use the terms anhydrous, hydrated and water of crystallisation calculate empirical and molecular formulae, using given data
2.4.1.1 reacting masses (from formulae and equations) including percentage yield calculations
2.4.1.2 volumes of gases (e.g. in the burning of hydrocarbons)
2.4.1.3 volumes and concentrations of solutions
2.4.1.4 limiting reagent and excess reagent (When performing calculations, candidates’ answers should reflect the number of significant figures given or asked for in the question. When rounding up or down, candidates should ensure that significant figures are neither lost unnecessarily nor used beyond what is justified (see also Mathematical requirements section).) deduce stoichiometric relationships from calculations such as those in 2.4.1 (1)–(4)
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
