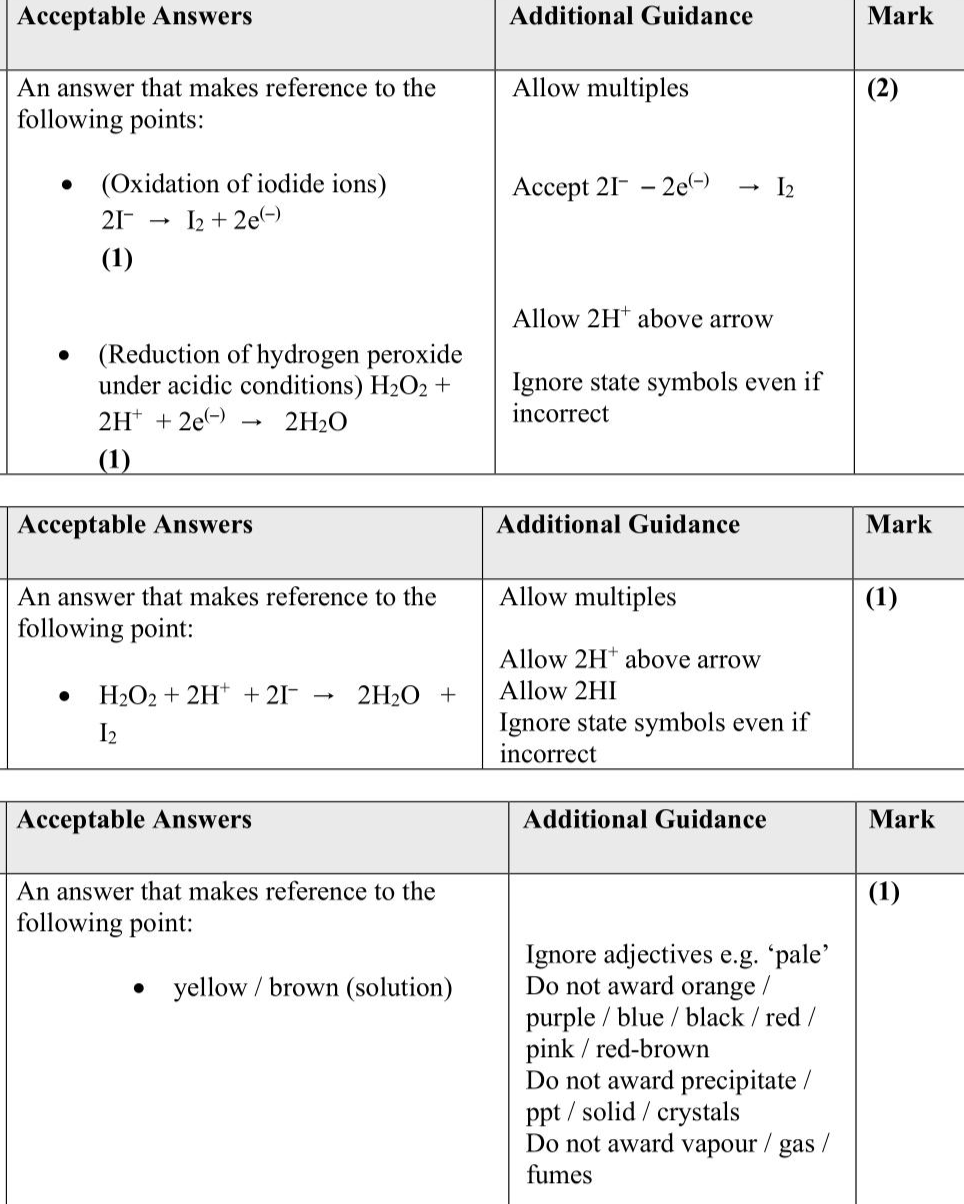
Seaweeds absorb iodide compounds from seawater. If seaweeds are dried and heated strongly, iodine can be obtained from the ash produced. Procedure Step 1 Heat the dried seaweed strongly to burn off any organic material. Step 2 Add the ash produced in Step 1 to $$\(25 \mathrm{~cm}^{3}\)$$ of deionised water and boil for 5 minutes. Step 3 Filter off the remaining solid, collecting the colourless filtrate containing iodide ions. Step 4 Add $$\(2 \mathrm{~cm}^{3}\)$$ of dilute sulfuric acid, followed by $$\(10 \mathrm{~cm}^{3}\)$$ of ' 20 volume' hydrogen peroxide solution, $$\(\mathrm{H}_{2} \mathrm{O}_{2}(\mathrm{aq})\)$$. Step 5 Extract the iodine formed in Step $$\(\mathbf{4}\)$$ using cyclohexane as the solvent. Step 6 Allow the cyclohexane to evaporate to leave behind iodine crystals. In Step 4, the iodide ions in the filtrate are oxidised to form iodine. The reaction takes place under acidic conditions and the hydrogen peroxide is reduced to form a single product, water. (i) Write half-equations for each of these changes. State symbols are not required. (2) Oxidation of iodide ions: Reduction of hydrogen peroxide under acidic conditions: (ii) Write the overall equation for the reaction between iodide ions and hydrogen peroxide solution under acidic conditions. State symbols are not required. (1) (iii) State the colour of the aqueous iodine solution formed in Step 4. (1) ................................................................................................................................................................................................................................................................................
Exam No:wch13-01-que-20240123 Year:2024 Question No:3(c)
Answer:
Knowledge points:
8.Redox Chemistry and Groups 1, 2 and 7
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
