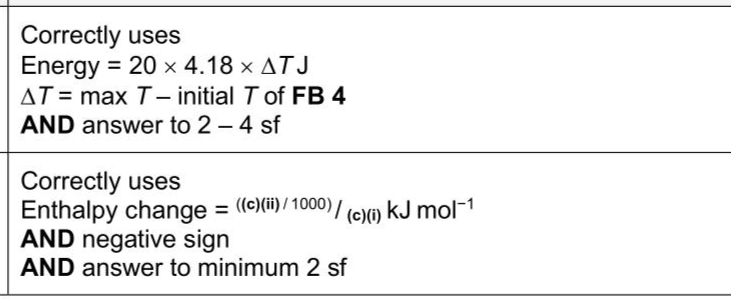
The reaction between an acid and an alkali is exothermic. You will carry out a neutralisation experiment to determine the enthalpy change involved. You will mix different volumes of an acid with a fixed volume of an alkali and measure the temperature rises that occur. FB 4 is aqueous sodium hydroxide, NaOH . FB 5 is $$\(2.00 \mathrm{~mol} \mathrm{dm}^{-3}\)$$ hydrochloric acid, HCl . (c)Calculations (i)Calculate the amount,in mol,of hydrochloric acid in the volume of FB $$\(\mathbf{5}\)$$ in(b)(ii). (If you were unable to determine an answer to(b)(ii),use $$\(5.10 \mathrm{~cm}^{3}\)$$ as the volume of FB 5.This may not be the correct answer.) amount of $$\(\mathrm{HCl}=\)$$ mol Deduce the amount,in mol,of sodium hydroxide in $$\(10.0 \mathrm{~cm}^{3}\)$$ of FB 4. amount of $$\(\mathrm{NaOH}=\)$$ mol [1] (ii)Calculate the energy change,in J ,when the amounts of reagents in(c)(i)neutralise each other.Show your working. energy change= J [1] (iii)Use your answer to(c)(ii)to calculate the enthalpy change,in $$\(\mathrm{kJ} \mathrm{mol}^{-1}\)$$ ,when one mole of FB 4 is neutralised by one mole of FB 5. enthalpy change= $$\(\mathrm{kJ} \mathrm{mol}^{-1}\)$$ [1] sign value [Total:12] For each test you should record all your observations in the spaces provided. Examples of observations include: - colour changes seen - the formation of any precipitate and its solubility (where appropriate) in an excess of the reagent added - the formation of any gas and its identification (where appropriate) by a suitable test. You should record clearly at what stage in a test an observation is made. Where no change is observed, you should write 'no change'. Where reagents are selected for use in a test, the name or correct formula of the element or compound must be given. If any solution is warmed, a boiling tube must be used. If a solid is heated, a hard-glass test-tube must be used. Rinse and reuse test-tubes and boiling tubes where possible. No additional tests should be attempted.
Exam No:9701_s25_qp_34 Year:2025 Question No:2(c)
Answer:

Knowledge points:
2.2.1 define and use the term mole in terms of the Avogadro constant
2.4.1.1 reacting masses (from formulae and equations) including percentage yield calculations
2.4.1.2 volumes of gases (e.g. in the burning of hydrocarbons)
2.4.1.3 volumes and concentrations of solutions
2.4.1.4 limiting reagent and excess reagent (When performing calculations, candidates’ answers should reflect the number of significant figures given or asked for in the question. When rounding up or down, candidates should ensure that significant figures are neither lost unnecessarily nor used beyond what is justified (see also Mathematical requirements section).) deduce stoichiometric relationships from calculations such as those in 2.4.1 (1)–(4)
5.1.1 understand that chemical reactions are accompanied by enthalpy changes and these changes can be exothermic (ΔH is negative) or endothermic (ΔH is positive)
5.1.2 construct and interpret a reaction pathway diagram, in terms of the enthalpy change of the reaction and of the activation energy
5.1.3.1 standard conditions (this syllabus assumes that these are 298 K and 101 kPa) shown by
5.1.3.2 enthalpy change with particular reference to: reaction, , combustion, , neutralisation,
5.1.4 understand that energy transfers occur during chemical reactions because of the breaking and making of chemical bonds
5.1.5 use bond energies (ΔH positive, i.e. bond breaking) to calculate enthalpy change of reaction
5.1.6 understand that some bond energies are exact and some bond energies are averages
5.1.7 calculate enthalpy changes from appropriate experimental results, including the use of the relationships q = mcΔT and ΔH = –mcΔT/n
7.2.1 state the names and formulae of the common acids, limited to hydrochloric acid, HC/, sulfuric acid, ethanoic acid,
7.2.10 select suitable indicators for acid-alkali titrations, given appropriate data
7.2.2 state the names and formulae of the common alkalis, limited to sodium hydroxide, NaOH, potassium hydroxide, KOH, ammonia,
7.2.3 describe the Brønsted–Lowry theory of acids and bases
7.2.4 describe strong acids and strong bases as fully dissociated in aqueous solution and weak acids and weak bases as partially dissociated in aqueous solution
7.2.5 appreciate that water has pH of 7, acid solutions pH of below 7 and alkaline solutions pH of above 7
7.2.6 explain qualitatively the differences in behaviour between strong and weak acids including the reaction with a reactive metal and difference in pH values by use of a pH meter, universal indicator or conductivity
7.2.7 understand that neutralisation reactions occur when
7.2.8 understand that salts are formed in neutralisation reactions
7.2.9 sketch the pH titration curves of titrations using combinations of strong and weak acids with strong and weak alkalis
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
