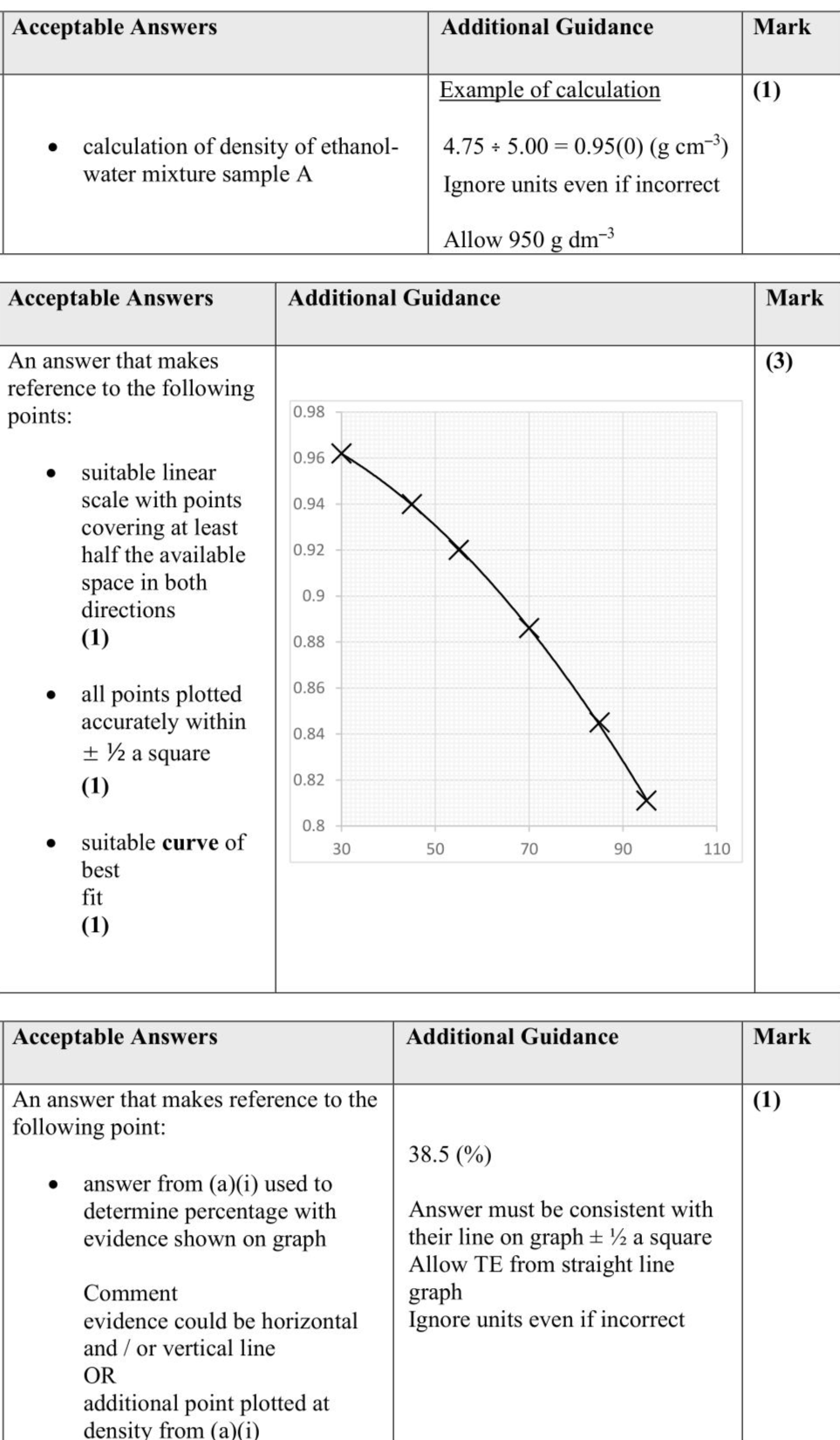
This question is about experiments involving ethanol. Ethanol and water mix in all proportions. The percentage of ethanol by volume in ethanol-water mixtures can be found by comparing the density of the mixture to the densities of ethanol-water mixtures of known composition, at a constant temperature. (i) Calculate the density of an ethanol-water mixture, sample $$\(\mathbf{A}, 5.00 \mathrm{~cm}^{3}\)$$ of which has a mass of $$\(4.75 \mathrm{~g}\)$$. (1) (ii) Plot a graph of density against the percentage of ethanol by volume. (3) (iii) Determine the percentage of ethanol by volume in sample $$\(\mathbf{A}\)$$ using your answer to (a)(i) and the graph in (a)(ii). Show your working on the graph. (1)
Exam No:wch13-01-que-20240123 Year:2024 Question No:4(a)
Answer:
Knowledge points:
1.Formulae, Equations and Amount of Substance
7.Intermolecular Forces
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
