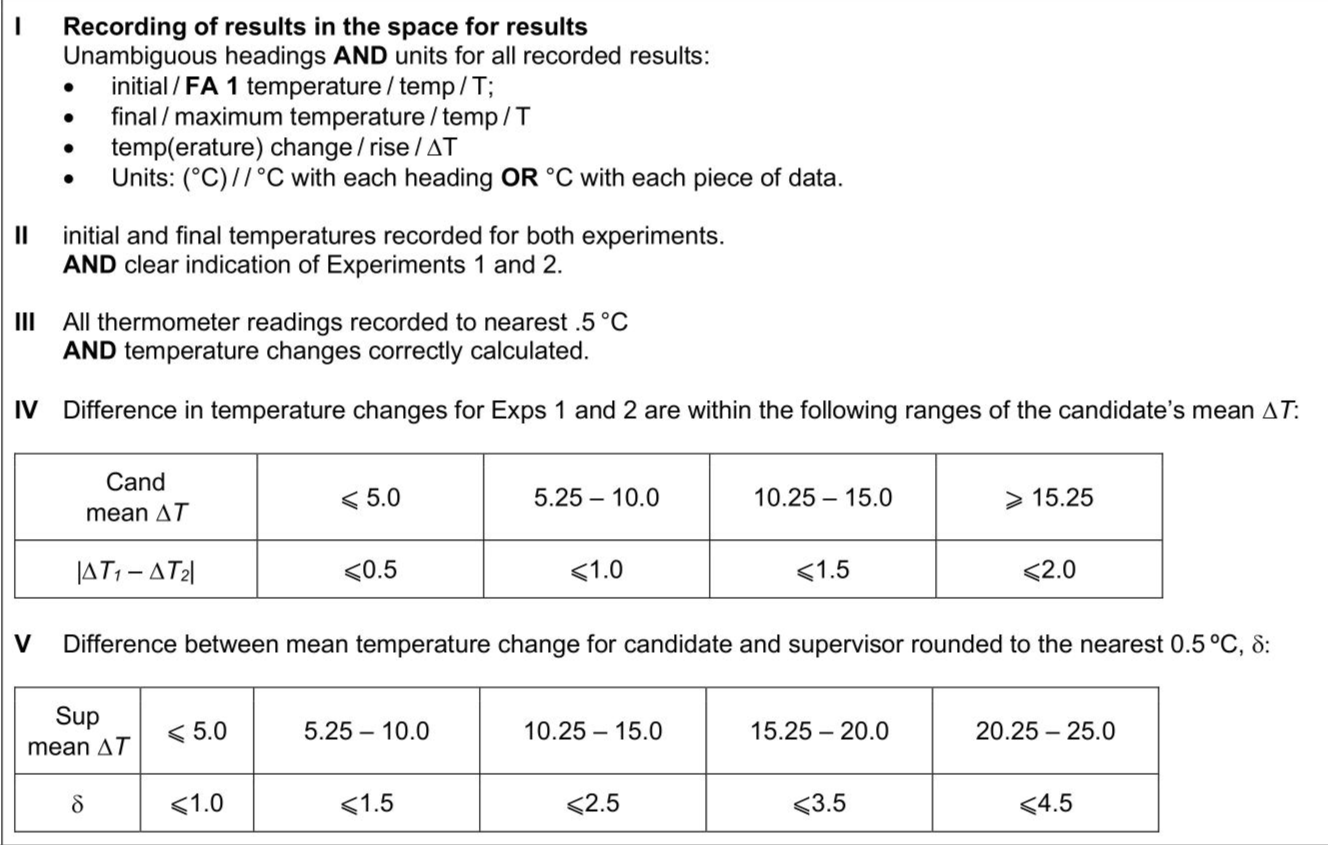
You will now determine the concentration of a solution of hydrogen peroxide by a different method. Hydrogen peroxide decomposes slowly into water and oxygen at room temperature. This reaction is exothermic. When a catalyst is added, the decomposition is fast and there is a measurable temperature rise. $$\[ \mathrm{H}_{2} \mathrm{O}_{2}(\mathrm{aq}) \rightarrow \mathrm{H}_{2} \mathrm{O}(\mathrm{l})+\frac{1}{2} \mathrm{O}_{2}(\mathrm{~g}) \quad \Delta H=-98.2 \mathrm{~kJ} \mathrm{~mol}^{-1} \]$$ FA 1 is aqueous hydrogen peroxide, $$\(\mathrm{H}_{2} \mathrm{O}_{2}\)$$. FA 5 is manganese(IV) oxide, $$\(\mathrm{MnO}_{2}\)$$. - Support one of the cups in the $$\(250 \mathrm{~cm}^{3}\)$$ beaker. - Use the $$\(50 \mathrm{~cm}^{3}\)$$ measuring cylinder to add $$\(25.0 \mathrm{~cm}^{3}\)$$ of FA 1 to the cup. - Place the thermometer in the FA 1 and tilt the cup, if necessary, so that the bulb of the thermometer is fully covered. Record the temperature in the space for results. - Add a heaped spatula measure of FA 5 to the solution in the cup. - Stir constantly until the maximum temperature is reached. Record this temperature. - Calculate and record the temperature rise. - Rinse and dry the thermometer. - Support the second cup in the $$\(250 \mathrm{~cm}^{3}\)$$ beaker. - Use the $$\(50 \mathrm{~cm}^{3}\)$$ measuring cylinder to add $$\(40.0 \mathrm{~cm}^{3}\)$$ of FA 1 to the second cup. - Measure and record the initial temperature of the solution. - Add a heaped spatula measure of FA 5 to the solution in the second cup. - Stir constantly until the maximum temperature is reached. Record this temperature. - Calculate and record the temperature rise.
Exam No:9701_s25_qp_37 Year:2025 Question No:2(a)
Answer:
Knowledge points:
5.1.1 understand that chemical reactions are accompanied by enthalpy changes and these changes can be exothermic (ΔH is negative) or endothermic (ΔH is positive)
5.1.2 construct and interpret a reaction pathway diagram, in terms of the enthalpy change of the reaction and of the activation energy
5.1.3.1 standard conditions (this syllabus assumes that these are 298 K and 101 kPa) shown by
5.1.3.2 enthalpy change with particular reference to: reaction, , combustion, , neutralisation,
5.1.4 understand that energy transfers occur during chemical reactions because of the breaking and making of chemical bonds
5.1.5 use bond energies (ΔH positive, i.e. bond breaking) to calculate enthalpy change of reaction
5.1.6 understand that some bond energies are exact and some bond energies are averages
5.1.7 calculate enthalpy changes from appropriate experimental results, including the use of the relationships q = mcΔT and ΔH = –mcΔT/n
8.3.1.1 explain that, in the presence of a catalyst, a reaction has a different mechanism, i.e. one of lower activation energy
8.3.1.2 explain this catalytic effect in terms of the Boltzmann distribution
8.3.1.3 construct and interpret a reaction pathway diagram, for a reaction in the presence and absence of an effective catalyst
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
