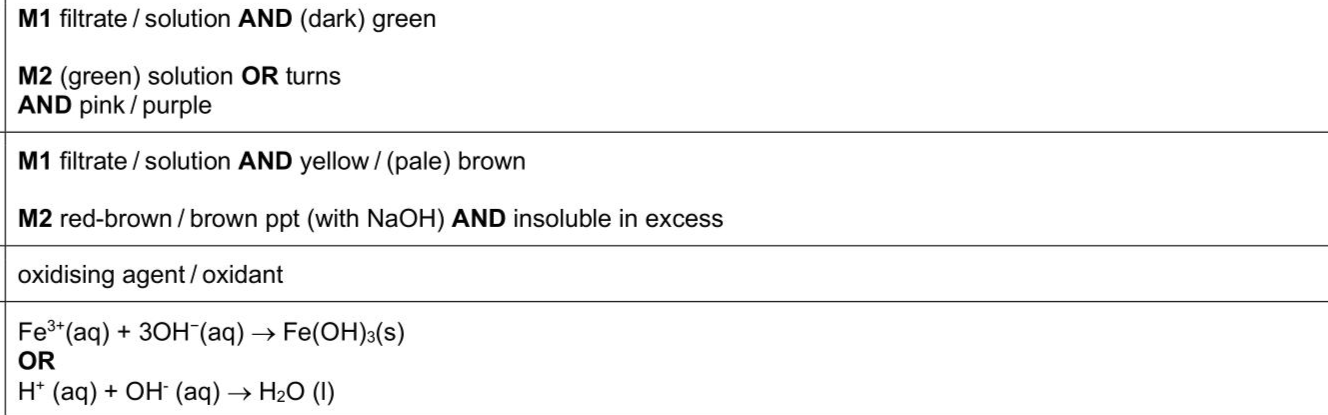
(a)(i)Transfer a 2 cm depth of FA 2, $$\(\mathrm{KMnO}_{4}\)$$ ,into a test-tube.Add the same volume of aqueous sodium hydroxide followed by a small spatula measure of $$\(\mathbf{F A 5}, \mathrm{MnO}_{2}\)$$ .Stir for approximately 30 seconds then filter the mixture into a second test-tube. observations . Add dilute sulfuric acid to the filtrate until no further change. observations . [2] (ii)Transfer a 1 cm depth of aqueous iron(II)sulfate into a boiling tube.Add the same depth of dilute sulfuric acid followed by a very small spatula measure of $$\(\mathbf{F A 5}, \mathrm{MnO}_{2}\)$$ .Carefully warm the mixture using a Bunsen burner for about 20 seconds.Filter the warm mixture into a test-tube. observations . Add aqueous sodium hydroxide dropwise to the filtrate until no further change. observations . [2] (iii) Suggest a conclusion about the chemical behaviour of FA 5 using your observations in (a)(ii). FA 5 is acting as ...................................................................................................... (iv) Write an ionic equation for the reaction between aqueous sodium hydroxide and the filtrate in (a)(ii). Include state symbols. .................................................................................................................................
Exam No:9701_s25_qp_33 Year:2025 Question No:3(a)
Answer:
Knowledge points:
3.2.1 define ionic bonding as the electrostatic attraction between oppositely charged ions (positively charged cations and negatively charged anions) describe ionic bonding including the examples of sodium chloride, magnesium oxide and calcium fluoride
6.1.1 calculate oxidation numbers of elements in compounds and ions
6.1.2 use changes in oxidation numbers to help balance chemical equations
6.1.3 explain and use the terms redox, oxidation, reduction and disproportionation in terms of electron transfer and changes in oxidation number
6.1.4 explain and use the terms oxidising agent and reducing agent
6.1.5 use a Roman numeral to indicate the magnitude of the oxidation number of an element
8.3.1.1 explain that, in the presence of a catalyst, a reaction has a different mechanism, i.e. one of lower activation energy
8.3.1.2 explain this catalytic effect in terms of the Boltzmann distribution
8.3.1.3 construct and interpret a reaction pathway diagram, for a reaction in the presence and absence of an effective catalyst
9.3.1 predict the characteristic properties of an element in a given group by using knowledge of chemical periodicity
9.3.2 deduce the nature, possible position in the Periodic Table and identity of unknown elements from given information about physical and chemical properties
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
