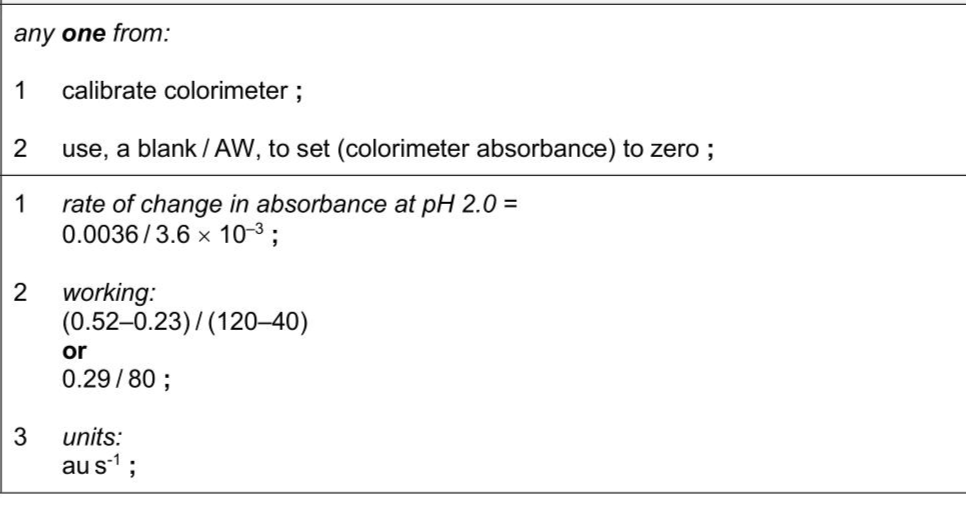
Pepsin is an enzyme that is present in gastric juice. Gastric juice is secreted into the stomach of humans and many other animals. Egg albumen (egg white) contains a high proportion of protein. $$\(10 \%\)$$ albumen solution is a cloudy-white colour. This becomes colourless when pepsin is added to the $$\(10 \%\)$$ albumen solution. A student used a colorimeter to follow the progress of the hydrolysis of protein by pepsin. The student used a blue filter in the colorimeter. Outline one other step the student should carry out to prepare the colorimeter so that correct measurements of absorbance can be obtained. . . (ii) After preparing the colorimeter, the student: - made a pH 2.0 albumen solution by mixing $$\(10 \mathrm{~cm}^{3}\)$$ of $$\(10 \%\)$$ albumen solution with $$\(10 \mathrm{~cm}^{3}\)$$ of pH 2.0 buffer solution - added $$\(3.0 \mathrm{~cm}^{3}\)$$ of the pH 2.0 albumen solution to a colorimeter tube - added a small volume of pepsin solution to the colorimeter tube - immediately placed the colorimeter tube in the colorimeter - recorded the absorbance of the mixture every 20 seconds for 5 minutes - calculated the rate of change in absorbance as a measure of the rate of protein hydrolysis. The results are shown in Fig. 1.2.
Exam No:9700_s25_qp_51 Year:2025 Question No:1(a)
Answer:
Knowledge points:
3.1.1 state that enzymes are globular proteins that catalyse reactions inside cells (intracellular enzymes) or are secreted to catalyse reactions outside cells (extracellular enzymes)
3.1.2 explain the mode of action of enzymes in terms of an active site, enzyme–substrate complex, lowering of activation energy and enzyme specificity, including the lock-and-key hypothesis and the induced-fit hypothesis
3.1.3 investigate the progress of enzyme-catalysed reactions by measuring rates of formation of products using catalase and rates of disappearance of substrate using amylase
3.1.4 outline the use of a colorimeter for measuring the progress of enzyme-catalysed reactions that involve colour changes
3.2.1.1 temperature
3.2.1.2 pH (using buffer solutions)
3.2.1.3 enzyme concentration
3.2.1.4 substrate concentration
3.2.1.5 inhibitor concentration
3.2.2 explain that the maximum rate of reaction (Vmax) is used to derive the Michaelis-Menten constant (Km) which is used to compare the affinity of different enzymes for their substrates
3.2.3 explain the effects of inhibitors, both competitive and non- competitive, on the rate of enzyme activity
3.2.4 investigate the difference in activity between an enzyme immobilised in alginate and the same enzyme free in solution, and state the advantages of using immobilised enzymes
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
