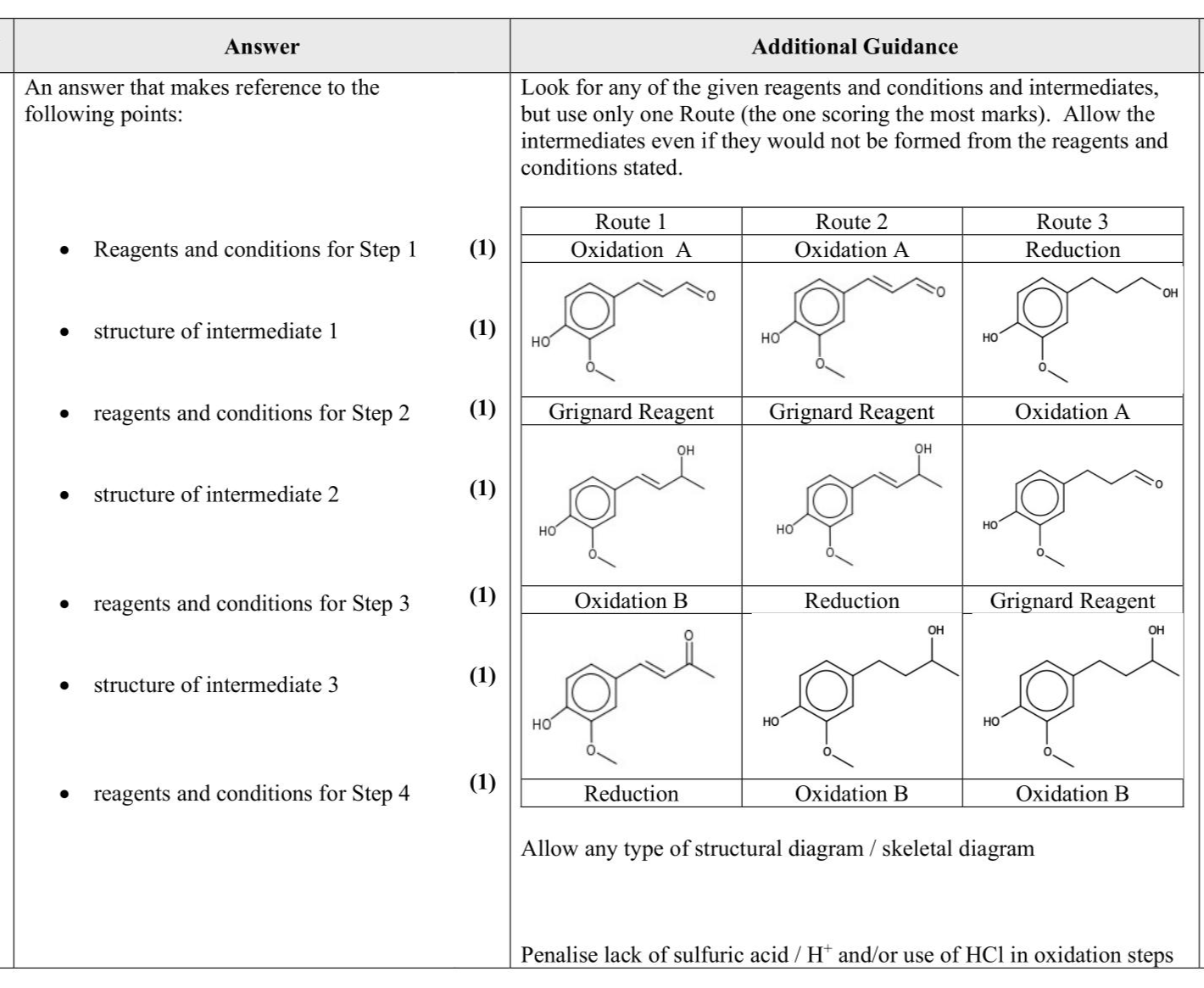
Cooking fresh ginger converts gingerol into zingerone, which is less pungent and has a sweeter flavour. Zingerone can be formed in a four-step synthesis from coniferyl alcohol. Step $$\(\mathbf{2}\)$$ in the synthesis involves a Grignard Reagent, while Steps 1, $$\(\mathbf{3}\)$$ and $$\(\mathbf{4}\)$$ are redox reactions. The synthesis is shown with the structures of the intermediate compounds incomplete. Complete this four-step synthesis of zingerone from coniferyl alcohol. Include in your answer completed structures of the intermediate compounds and the reagents and conditions required. (7)
Exam No:wch15-01-que-20240113 Year:2024 Question No:15(b)
Answer:
Knowledge points:
20.Organic Synthesis
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
