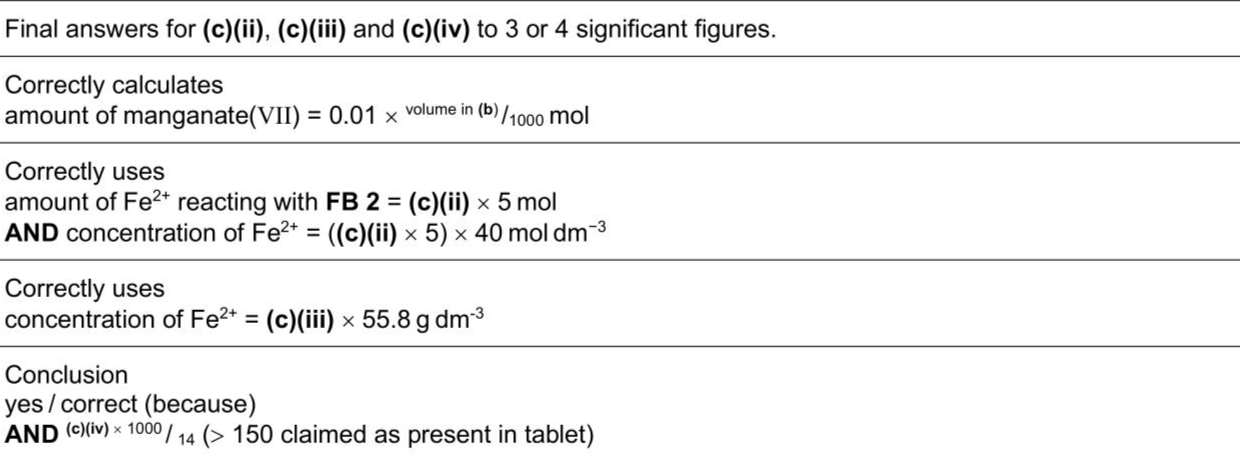
Iron is an element that is essential in the human diet. Some people need to take iron supplement tablets to ensure an adequate intake of iron. You will investigate the mass of iron in an iron supplement tablet by titrating a solution with potassium manganate(VII). $$\[ \begin{gathered} \mathrm{Fe}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{e}^{-} \\ \mathrm{MnO}_{4}^{-}(\mathrm{aq})+8 \mathrm{H}^{+}(\mathrm{aq})+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}(\mathrm{aq})+4 \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \end{gathered} \]$$ FB 1 is an aqueous solution of iron supplement tablets made by dissolving 14 tablets in $$\(1.00 \mathrm{dm}^{3}\)$$ of solution. The iron in each tablet is iron(II) sulfate, $$\(\mathrm{FeSO}_{4} \bullet 7 \mathrm{H}_{2} \mathrm{O}\)$$. FB 2 is $$\(0.0100 \mathrm{~mol} \mathrm{dm}^{-3}\)$$ acidified aqueous potassium manganate(VII), $$\(\mathrm{KMnO}_{4}\)$$. FB 3 is dilute sulfuric acid, $$\(\mathrm{H}_{2} \mathrm{SO}_{4}\)$$. Calculations (i) Give your answers to (c)(ii), (c)(iii) and (c)(iv) to an appropriate number of significant figures. (ii) Calculate the amount, in mol, of manganate(VII) ions in the volume of FB 2 in (b). amount of $$\(\mathrm{MnO}_{4}{ }^{-}=\)$$ mol (iii) Use your answer to (c)(ii) and the equations at the start of the question to calculate the concentration, in $$\(\mathrm{mol} \mathrm{dm}^{-3}\)$$, of iron(II) ions in FB 1. concentration of $$\(\mathrm{Fe}^{2+}=\)$$ $$\(\mathrm{mol} \mathrm{dm}^{-3}\)$$ (iv) Use your answer to (c)(iii) to calculate the concentration, in $$\(\mathrm{gdm}^{-3}\)$$, of iron(II) ions in FB 1. concentration of $$\(\mathrm{Fe}^{2+}=\)$$ $$\(\mathrm{g} \mathrm{dm}^{-3}\)$$ (v) The manufacturer of the iron supplement tablets used to make FB 1 claims that each tablet contains a minimum of 150 mg of $$\(\mathrm{Fe}^{2+}\)$$. Use your answer to (c)(iv) and the information given about FB 1 to determine whether this claim is correct. Show your working.
Exam No:9701_s25_qp_34 Year:2025 Question No:1(c)
Answer:
Knowledge points:
2.2.1 define and use the term mole in terms of the Avogadro constant
2.4.1.1 reacting masses (from formulae and equations) including percentage yield calculations
2.4.1.2 volumes of gases (e.g. in the burning of hydrocarbons)
2.4.1.3 volumes and concentrations of solutions
2.4.1.4 limiting reagent and excess reagent (When performing calculations, candidates’ answers should reflect the number of significant figures given or asked for in the question. When rounding up or down, candidates should ensure that significant figures are neither lost unnecessarily nor used beyond what is justified (see also Mathematical requirements section).) deduce stoichiometric relationships from calculations such as those in 2.4.1 (1)–(4)
6.1.1 calculate oxidation numbers of elements in compounds and ions
6.1.2 use changes in oxidation numbers to help balance chemical equations
6.1.3 explain and use the terms redox, oxidation, reduction and disproportionation in terms of electron transfer and changes in oxidation number
6.1.4 explain and use the terms oxidising agent and reducing agent
6.1.5 use a Roman numeral to indicate the magnitude of the oxidation number of an element
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
