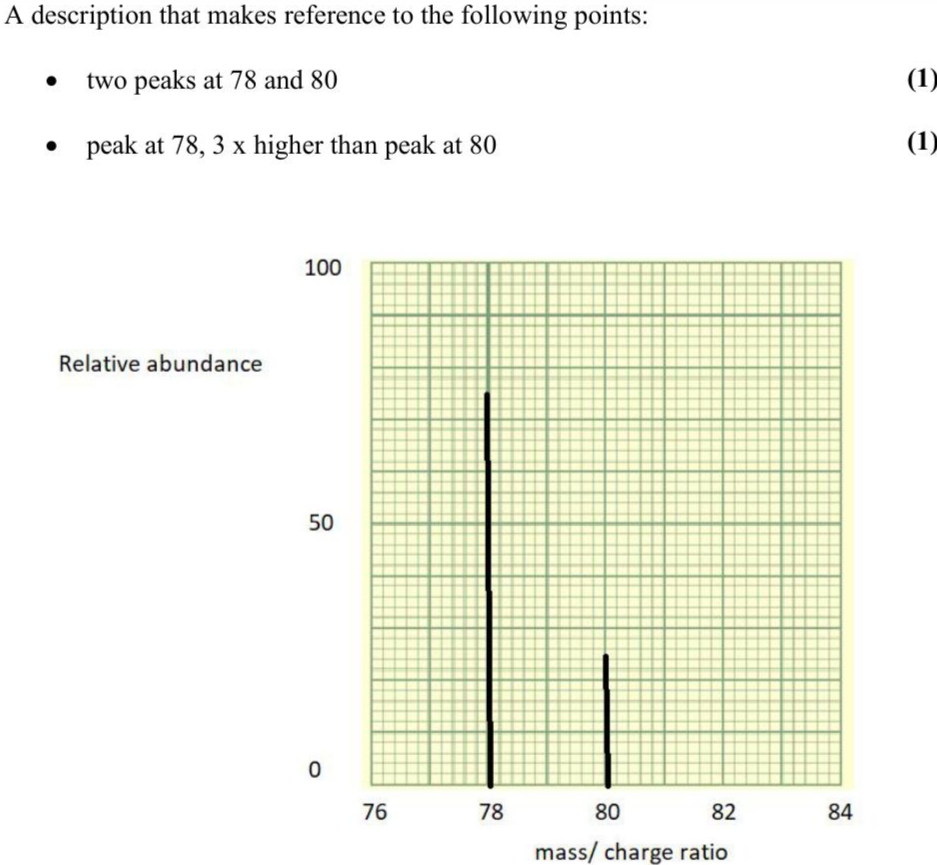
This question is about 2-Chloropropane. 2-Chloropropane has a relative molecular mass of $$\(78.5 \mathrm{~g} \mathrm{~mol}^{-1}\)$$. Chlorine has two common isotopes, $$\({ }^{35} \mathrm{Cl}\)$$ and $$\({ }^{37} \mathrm{Cl}\)$$. There are three times more $$\({ }^{35} \mathrm{Cl}\)$$ atoms than $$\({ }^{37} \mathrm{Cl}\)$$ atoms. The main isotope of hydrogen is $$\({ }^{1} \mathrm{H}\)$$ and that of carbon is $$\({ }^{12} \mathrm{C}\)$$. The diagram shows a mass spectrum grid. Draw the peaks for the molecular ions of 2-Chloropropane resulting from these isotopes. (2)
Exam No:wch11-01-que-20240111 Year:2024 Question No:19(a)
Answer:
Knowledge points:
2.Atomic Structure and the Periodic Table
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
