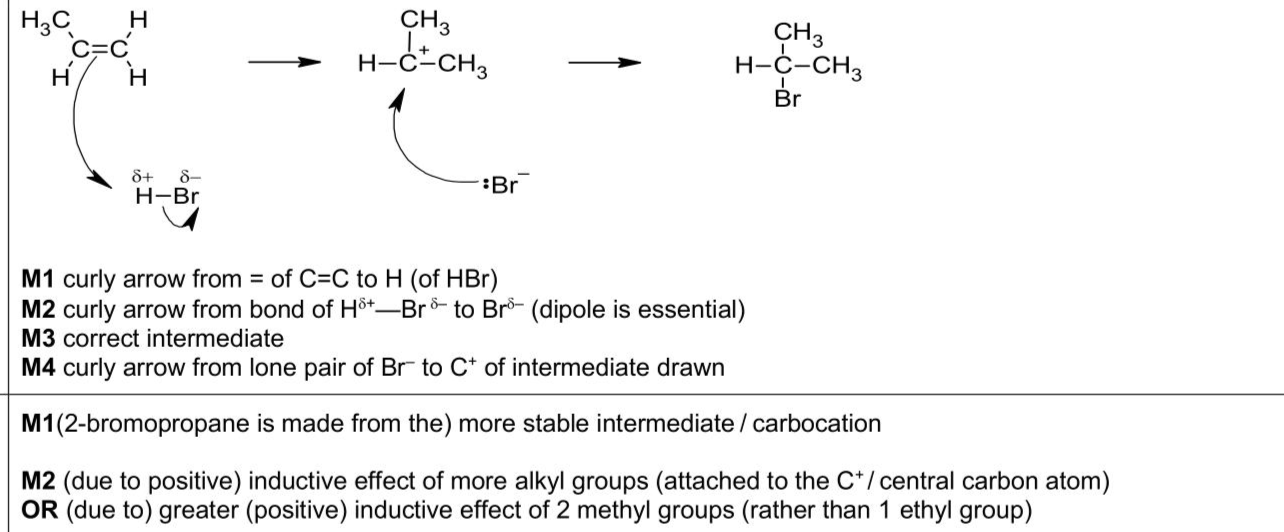
(a) $$\(\mathrm{C}_{3} \mathrm{H}_{6}\)$$ reacts with $$\(\mathrm{HBr}(\mathrm{g})\)$$ in an addition reaction. (i)Define addition reaction. . (ii)Complete Fig. 3.1 to show the mechanism for the addition reaction between $$\(\mathrm{C}_{3} \mathrm{H}_{6}\)$$ and HBr to produce 2-bromopropane.Include charges,dipoles,lone pairs of electrons and curly arrows,as appropriate. (iii)Explain why the major product of this reaction is 2-bromopropane rather than NIOCYW SIH $$\(\perp\)$$ NI ヨHYM $$\(\perp\)$$ ON OO NIOYVW SIH $$\(\perp\)$$ NI ヨHYM $$\(\perp\)$$ ON OO [4] 1-bromopropane. . . [2]
Exam No:9701_s25_qp_22 Year:2025 Question No:3(a)
Answer:

Knowledge points:
13.2.1.1 homologous series
13.2.1.2 saturated and unsaturated
13.2.1.3 homolytic and heterolytic fission
13.2.1.4 free radical, initiation, propagation, termination (the use of arrows to show movement of single electrons is not required)
13.2.1.5 nucleophile, electrophile, nucleophilic, electrophilic
13.2.1.6 addition, substitution, elimination, hydrolysis, condensation
13.2.1.7 oxidation and reduction (in equations for organic redox reactions, the symbol [O] can be used to represent one atom of oxygen from an oxidising agent and the symbol [H] one atom of hydrogen from a reducing agent)
13.2.2.1 free-radical substitution
13.2.2.2 electrophilic addition
13.2.2.3 nucleophilic substitution
13.2.2.4 nucleophilic addition (in organic reaction mechanisms, the use of curly arrows to represent movement of electron pairs is expected; the arrow should begin at a bond or a lone pair of electrons)
13.3.1 describe organic molecules as either straight-chained, branched or cyclic
13.3.2 describe and explain the shape of, and bond angles in, molecules containing sp, sp2 and sp3 hybridised atoms
13.3.3 describe the arrangement of σ and π bonds in molecules containing sp, sp2 and sp3 hybridised atoms
13.3.4 understand and use the term planar when describing the arrangement of atoms in organic molecules, for example ethene
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
