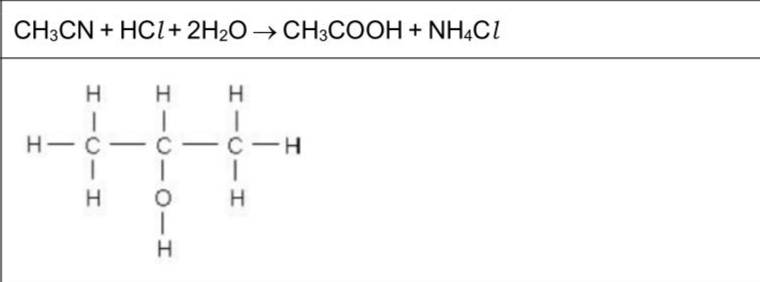
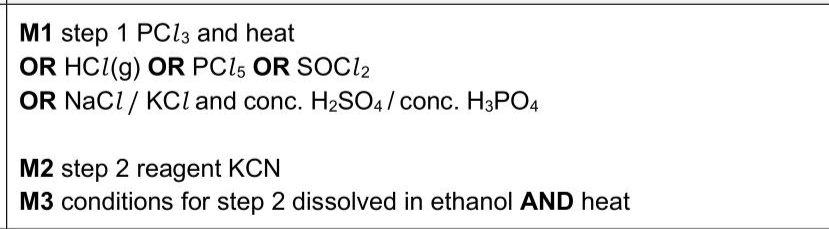
The skeletal formula of $$\(\mathbf{E}\)$$ is shown in Fig.4.1. $$\(F\)$$ is an isomer of $$\(E\)$$. F reacts with $$\(\mathrm{HCl}(\mathrm{aq})\)$$ to produce $$\(\mathbf{G}\)$$ and $$\(\mathbf{H}\)$$. $$\(\mathbf{G}\)$$ is a secondary alcohol. $$\(\mathbf{H}\)$$ is also produced when $$\(\mathrm{HCl}(\mathrm{aq})\)$$ is added to $$\(\mathrm{CH}_{3} \mathrm{CN}\)$$. (i) Construct an equation for the acid hydrolysis of $$\(\mathrm{CH}_{3} \mathrm{CN}\)$$ with $$\(\mathrm{HCl}(\mathrm{aq})\)$$. ................................................................................................................................. (ii) Draw the displayed formula of $$\(\mathbf{G}\)$$. (iii) $$\(\mathrm{CH}_{3} \mathrm{OH}\)$$ is used to make $$\(\mathrm{CH}_{3} \mathrm{CN}\)$$ in a two-step process, as shown in Fig. 4.2. State the reagents and conditions required for steps 1 and 2. step 1 step 2
Exam No:9701_s25_qp_24 Year:2025 Question No:4(d)
Answer:
Knowledge points:
15.1.1.1 the free-radical substitution of alkanes by in the presence of ultraviolet light, as exemplified by the reactions of ethane
15.1.1.2 electrophilic addition of an alkene with a halogen, or hydrogen halide, HX(g), at room temperature
15.1.1.3 substitution of an alcohol, e.g. by reaction with HX or KBr with ; or with and heat; or with ; or with
15.1.2 classify halogenoalkanes into primary, secondary and tertiary
15.1.3.1 the reaction with NaOH(aq) and heat to produce an alcohol
15.1.3.2 the reaction with KCN in ethanol and heat to produce a nitrile
15.1.3.3 the reaction with in ethanol heated under pressure to produce an amine
15.1.3.4 the reaction with aqueous silver nitrate in ethanol as a method of identifying the halogen present as exemplified by bromoethane
15.1.4 describe the elimination reaction with NaOH in ethanol and heat to produce an alkene as exemplified by bromoethane
15.1.5 describe themechanisms of nucleophilic substitution in halogenoalkanes including the inductive effects of alkyl groups
15.1.6 recall that primary halogenoalkanes tend to react via the mechanism; tertiary halogenoalkanes via the mechanism; and secondary halogenoalkanes by a mixture of the two, depending on structure
15.1.7 describe and explain the different reactivities of halogenoalkanes (with particular reference to the relative strengths of the C–X bonds as exemplified by the reactions of halogenoalkanes with aqueous silver nitrates)
16.1.1.1 electrophilic addition of steam to an alkene, catalyst
16.1.1.2 reaction of alkenes with cold dilute acidified potassium manganate(VII) to form a diol
16.1.1.3 substitution of a halogenoalkane using NaOH(aq) and heat
16.1.1.4 reduction of an aldehyde or ketone using
16.1.1.5 reduction of a carboxylic acid using
16.1.1.6 hydrolysis of an ester using dilute acid or dilute alkali and heat
16.1.2.1 the reaction with oxygen (combustion)
16.1.2.2 substitution to halogenoalkanes
16.1.2.3 the reaction with Na(s)
16.1.2.4 oxidation with acidified or acidified to:
16.1.2.5 carbonyl compounds by distillation
16.1.2.6 carboxylic acids by refluxing (primary alcohols give aldehydes which can be further oxidised to carboxylic acids, secondary alcohols give ketones, tertiary alcohols cannot be oxidised)
16.1.2.7 dehydration to an alkene, by using a heated catalyst, e.g. or a concentrated acid
16.1.2.8 formation of esters by reaction with carboxylic acids and concentrated or as catalyst as exemplified by ethanol
16.1.3.1 classify alcohols as primary, secondary and tertiary alcohols, to include examples with more than one alcohol group
16.1.3.2 state characteristic distinguishing reactions, e.g. mild oxidation with acidified K2Cr2O7, colour change from orange to green
16.1.4 deduce the presence of a group in an alcohol, , from its reaction with alkaline (aq) to form a yellow precipitate of tri-iodomethane and an ion,
16.1.5 explain the acidity of alcohols compared with water
19.2.1.1 reaction of a halogenoalkane with KCN in ethanol and heat
19.2.2.1 the reaction of aldehydes and ketones with HCN, KCN as catalyst, and heat
19.2.3 describe the hydrolysis of nitriles with dilute acid or dilute alkali followed by acidification to produce a carboxylic acid
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
