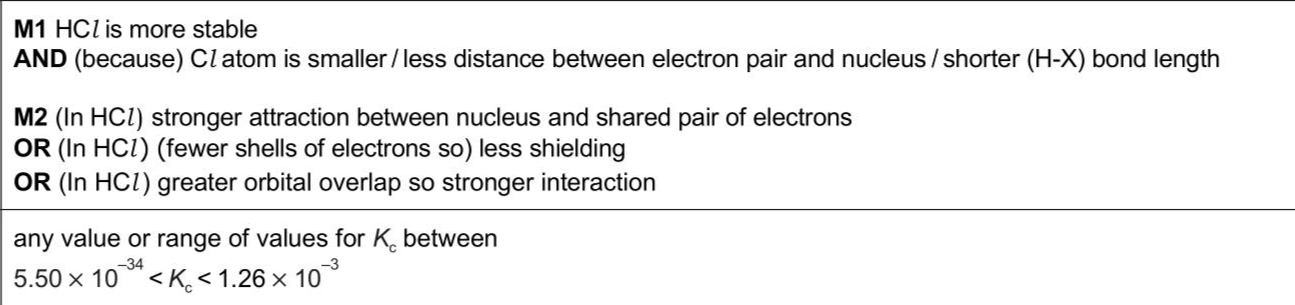
$$\(3 \quad \mathrm{H}_{2}(\mathrm{~g})\)$$ and $$\(\mathrm{I}_{2}(\mathrm{~g})\)$$ react to form $$\(\mathrm{HI}(\mathrm{g})\)$$ in a reversible reaction, as shown in equation 1. In three separate experiments, a student combines different amounts of two or more of the gases from equation 1. In each experiment, the gases are left to reach equilibrium at a given temperature. The value of $$\(K_{\mathrm{c}}\)$$ for the dissociation of HCl at 298 K is $$\(5.50 \times 10^{-34}\)$$. $$\[ 2 \mathrm{HCl}(\mathrm{~g}) \rightleftharpoons \mathrm{H}_{2}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g}) \quad K_{\mathrm{c}}=5.50 \times 10^{-34} \]$$ (i) Define covalent bond. . (ii) Describe and explain the relative thermal stabilities of the hydrogen halides HCl and HI . . . (iii) Use the data given in (c) and (d) to suggest a value for the equilibrium constant for the dissociation of $$\(\operatorname{HBr}(\mathrm{g})\)$$ at 298 K .
Exam No:9701_s25_qp_24 Year:2025 Question No:3(d)
Answer:
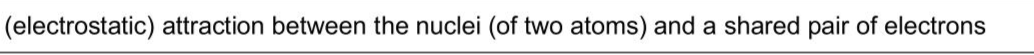
Knowledge points:
3.4.1.1.1 hydrogen,
3.4.1.1.10 ethene,
3.4.1.1.2 oxygen,
3.4.1.1.3 nitrogen,
3.4.1.1.4 chlorine
3.4.1.1.5 hydrogen chloride, HCl
3.4.1.1.6 carbon dioxide,
3.4.1.1.7 ammonia,
3.4.1.1.8 methane,
3.4.1.1.9 ethane,
3.4.1.2 understand that elements in period 3 can expand their octet including in the compounds sulfur dioxide, , phosphorus pentachloride, , and sulfur hexafluoride,
3.4.1.3 describe coordinate (dative covalent) bonding, including in the reaction between ammonia and hydrogen chloride gases to form the ammonium ion, , and in the molecule
3.4.2.1.1 $\sigma $ bonds are formed by direct overlap of orbitals between the bonding atoms
3.4.2.1.2 π bonds are formed by the sideways overlap of adjacent p orbitals above and below the σ bond
3.4.2.2 describe how the σ and π bonds form in molecules including
3.4.2.3 use the concept of hybridisation to describe sp, $\mathrm{sp}^{2}$ and $\mathrm{sp}^{3}$ orbitals
3.4.3.1.1 bond energy as the energy required to break one mole of a particular covalent bond in the gaseous state
3.4.3.1.2 bond length as the internuclear distance of two covalently bonded atoms
3.4.3.2 use bond energy values and the concept of bond length to compare the reactivity of covalent molecules
5.1.1 understand that chemical reactions are accompanied by enthalpy changes and these changes can be exothermic (ΔH is negative) or endothermic (ΔH is positive)
5.1.2 construct and interpret a reaction pathway diagram, in terms of the enthalpy change of the reaction and of the activation energy
5.1.3.1 standard conditions (this syllabus assumes that these are 298 K and 101 kPa) shown by
5.1.3.2 enthalpy change with particular reference to: reaction, , combustion, , neutralisation,
5.1.4 understand that energy transfers occur during chemical reactions because of the breaking and making of chemical bonds
5.1.5 use bond energies (ΔH positive, i.e. bond breaking) to calculate enthalpy change of reaction
5.1.6 understand that some bond energies are exact and some bond energies are averages
5.1.7 calculate enthalpy changes from appropriate experimental results, including the use of the relationships q = mcΔT and ΔH = –mcΔT/n
7.1.1.1 understand what is meant by a reversible reaction
7.1.1.2 understand what is meant by dynamic equilibrium in terms of the rate of forward and reverse reactions being equal and the concentration of reactants and products remaining constant
7.1.1.3 understand the need for a closed system in order to establish dynamic equilibrium
7.1.10 describe and explain the conditions used in the Haber process and the Contact process, as examples of the importance of an understanding of dynamic equilibrium in the chemical industry and the application of Le Chatelier’s principle
7.1.2 define Le Chatelier’s principle as: if a change is made to a system at dynamic equilibrium, the position of equilibrium moves to minimise this change
7.1.3 use Le Chatelier’s principle to deduce qualitatively (from appropriate information) the effects of changes in temperature, concentration, pressure or presence of a catalyst on a system at equilibrium
7.1.4 deduce expressions for equilibrium constants in terms of concentrations,
7.1.5 use the terms mole fraction and partial pressure
7.1.6 deduce expressions for equilibrium constants in terms of partial pressures,
7.1.7 use the expressions to carry out calculations (such calculations will not require the solving of quadratic equations)
7.1.8 calculate the quantities present at equilibrium, given appropriate data
7.1.9 state whether changes in temperature, concentration or pressure or the presence of a catalyst affect the value of the equilibrium constant for a reaction
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
