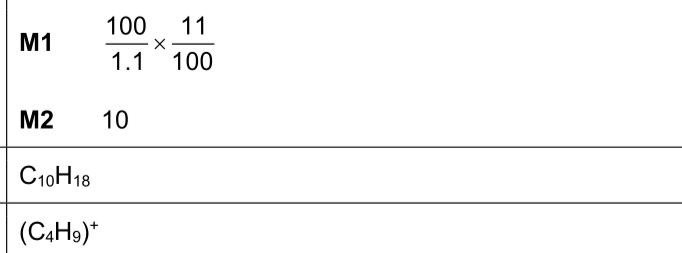
$$\(6 \quad \mathbf{P}, \mathbf{Q}\)$$ and $$\(\mathbf{R}\)$$ are three different hydrocarbon molecules. The mass spectrum of hydrocarbon $$\(\mathbf{R}\)$$ is recorded. Information about the two peaks with $$\(m / e\)$$ greater than 135 is shown in Fig. 6.1. (i) Use Fig. 6.1 to deduce the number of carbon atoms in a molecule of $$\(\mathbf{R}\)$$. Show your working. number of carbon atoms = .......................... (ii) Use Fig. 6.1 and your answer to (c)(i) to deduce the molecular formula of $$\(\mathbf{R}\)$$. (iii) Suggest the molecular formula of the fragment of $$\(\mathbf{R}\)$$ with $$\(m / e=57\)$$.
Exam No:9701_s25_qp_24 Year:2025 Question No:(c)
Answer:
Knowledge points:
22.2.1 analyse mass spectra in terms of m/e values and isotopic abundances (knowledge of the working of the mass spectrometer is not required)
22.2.2 calculate the relative atomic mass of an element given the relative abundances of its isotopes, or its mass spectrum
22.2.3 deduce the molecular mass of an organic molecule from the molecular ion peak in a mass spectrum
22.2.4 suggest the identity of molecules formed by simple fragmentation in a given mass spectrum
22.2.5 deduce the number of carbon atoms, n, in a compound using the M +1 peak and the formula
22.2.6 deduce the presence of bromine and chlorine atoms in a compound using the M +2 peak
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
