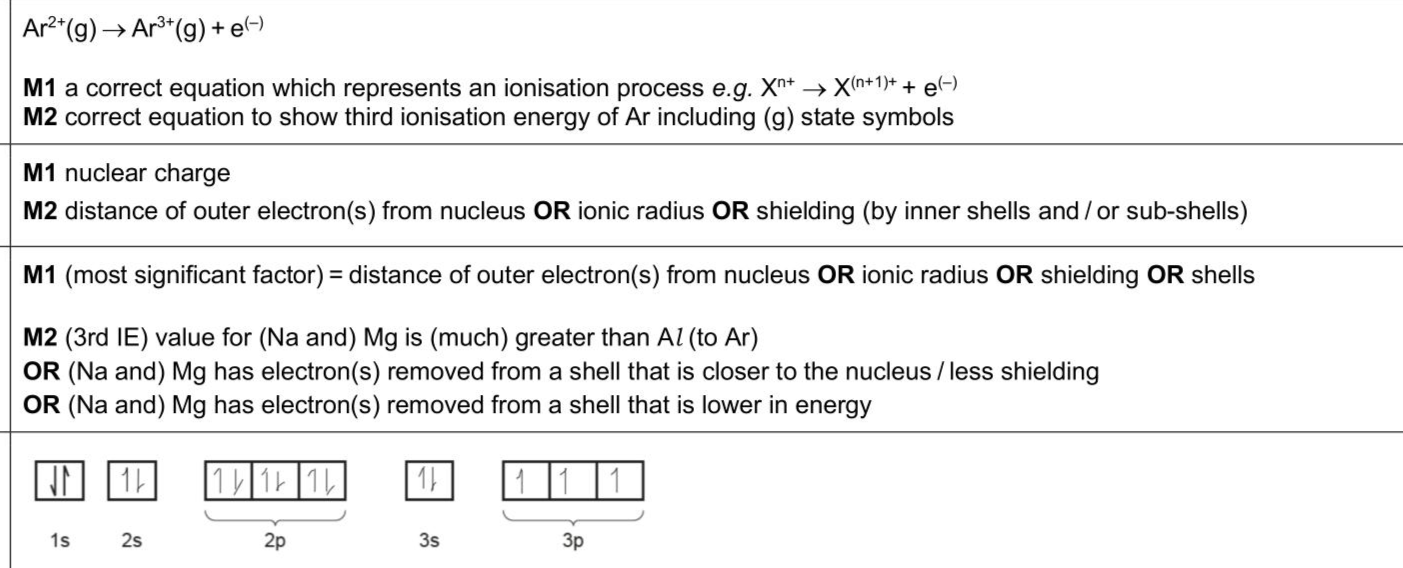
Elements in Period 3 of the Periodic Table show trends in their properties. The third ionisation energies of the Period 3 elements are shown in Fig. 1.2. (i) Write an equation, including state symbols, to represent the third ionisation energy of argon. (ii) The differences in the values for third ionisation energy shown in Fig. 1.2 are due to differences in the strength of attraction between the nucleus and the outer electron of each ion. State two factors that affect the strength of attraction between the nucleus and the outer electron. 1 2 (iii) Use Fig. 1.2 to suggest the most significant factor that determines the size of attraction between the nucleus and the outer electron. Explain your answer. . . (iv) In the third ionisation energy of argon, the ion produced has the electronic configuration $$\(1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{3}\)$$.
Exam No:9701_s25_qp_24 Year:2025 Question No:1(c)
Answer:
Knowledge points:
1.4.1 define and use the term first ionisation energy, IE
1.4.2 construct equations to represent first, second and subsequent ionisation energies
1.4.3 identify and explain the trends in ionisation energies across a period and down a group of the Periodic Table
1.4.4 identify and explain the variation in successive ionisation energies of an element
1.4.5 understand that ionisation energies are due to the attraction between the nucleus and the outer electron
1.4.6 explain the factors influencing the ionisation energies of elements in terms of nuclear charge, atomic/ionic radius, shielding by inner shells and sub-shells and spin-pair repulsion
1.4.7 deduce the electronic configurations of elements using successive ionisation energy data deduce the position of an element in the Periodic Table using successive ionisation energy data
Solution:
Download APP for more features
1. Tons of answers.
2. Smarter Al tools enhance your learning journey.
IOS
Download
Download
Android
Download
Download
Google Play
Download
Download
